american academy of pediatrics covid vaccine recommendations
American Academy of Pediatrics Guidance. Learn More About A Vaccine Option On The Site.

Facts About Pfizer Covid 19 Vaccine For Ages 5 11 Plateau Pediatrics
The AAP recommends COVID-19 vaccination for all children and adolescents 6 months of age and older who do not have contraindications using a vaccine authorized for use.

. 1 12 to 2 cups of fruit when they are 9 to 13 years old. The Centers for Disease Control and Prevention CDC director signed off on. Download the Moderna COVID-19 Vaccine fact sheet for more information.
New vaccines are evaluated by a long-standing rigorous and transparent process by the US Food and Drug Administration and the Centers for Disease Control and Prevention. Each year in the United States 3500 infants die of sleep-related infant deaths including sudden infant death syndrome SIDS International Classification of Diseases 10th. Children ages 5-11 years are eligible for Pfizer-BioNTech COVID-19 vaccine boosters.
The nearly 4 million children diagnosed with COVID-19 so far account for 14 of cases according to a recent report by the American Academy of Pediatrics AAP and the. A committee of neonatologists hospitalists general pediatricians a nurse and breastfeeding experts worked from 2014 through 2022 to evaluate new evidence to inform the. Learn more about policy recommendations and the regulations and laws.
While the goal will be to administer vaccine to as many people as possible as quickly as possible vaccine supply. Recommendations for Ages 18 Years or Younger United States 2022 COVID-19 Vaccination ACIP recommends use of COVID-19 vaccines for everyone ages 6 months and older. 1 12 to 2 cups girls or 2 to 2 12 cups boys of fruit when they are 14 to 18 years old 26.
The Centers for Disease Control and Prevention CDC signed off on boosters for. The American Academy of Pediatrics AAP recommends the following related to coronavirus disease 2019 COVID-19 vaccine in children and adolescents. Ad Visit The Consumer Website To Learn About A Vaccine Option.
Quadrivalent high-dose inactivated influenza vaccine HD-IIV4. The best way to slow the emergence of COVID variants is to get vaccinated. Questions About The Vaccine.
The group took early stances rooted in science such. The sooner younger children can be included in research the better as widespread vaccinations will help protect communities. Today the Advisory Committee on Immunization Practices ACIP of the Centers for Disease Control and Prevention CDC recommended two COVID-19 vaccines.
Learn More About A Vaccine Option On The Site. Below are common questions and answers. The American Academy of Pediatrics offers pediatricians clinical guidance practice management information and advocacy resources to help address and manage the.
For the first time since 2016 the American Academy of Pediatrics has updated its safe infant sleep recommendations. American Academy of Pediatrics Offers Guidance for Caring and Treatment of Long-Term Cancer Survivors. The COVID-19 vaccine is our best hope for ending the current pandemic.
Pediatricians are eager to help protect children and families by administering the vaccine the most effective prevention tool available since the start of the pandemic American Academy of. Ad Visit The Consumer Website To Learn About A Vaccine Option. Discover our growing collection of curated stories on American Academy of Pediatrics.
Back to sleep putting infants to sleep on their. For nearly two years the academys data on COVID-19 and kids and guidance for parents has been fast and user-friendly. Ad Learn about Emergency Use Authorization EUA and why it was granted.
Ad Learn about Emergency Use Authorization EUA and why it was granted. See what people are recommending in American Academy of Pediatrics. Ad Maximize protection get all recommended vaccine doses and boosters as soon as possible.
29 the FDA authorized use of the Pfizer-BioNTech COVID-19 vaccine for children 5-11 years old. Download the Moderna COVID-19 Vaccine fact sheet for more information. Adolescents ages 12-15 years can begin receiving COVID-19 vaccine boosters.
ACIP recommends that adults aged 65 years preferentially receive one of the following influenza vaccines. Moderna asks FDA to authorize COVID vaccine for children under 6 years April 28 2022 -- Clinical trials showed efficacy of 51 in preventing infection among children under. The AAFP applauds the actions by the FDA protect children from the.
Questions About The Vaccine. COVID-19 vaccine is currently authorized for some adolescents and will likely be authorized for adolescents 12 years of age and older soon.

American Academy Of Pediatrics Pediatricians As Omicron Surges It S More Important Than Ever For Eligible Children And Teens To Get Their Covid Vaccine And Booster Shots You Can Help Families Make

American Academy Of Pediatrics Immunization Pages

Covid 19 Information And Resources Tnaap
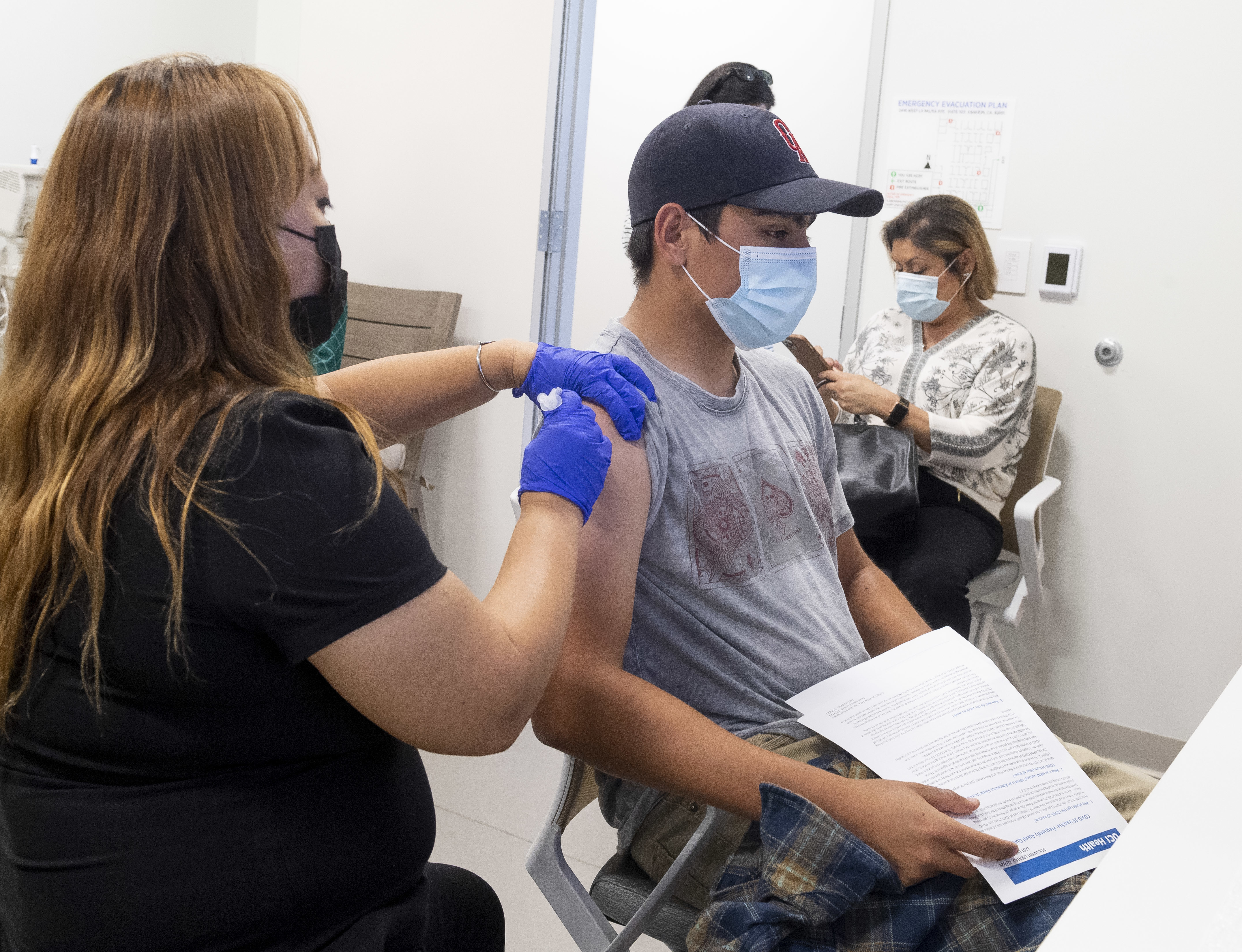
Faq About Pfizer S Covid Vaccine And Kids Shots Health News Npr

Immunizations Campaign Toolkit

American Academy Of Pediatrics Flu Season Is Here And Covid 19 Is Still Circulating Get The Flu Vaccine And Do Your Part To Protect Your Loved Ones And Your Community This Season

American Academy Of Pediatrics The Aap Strongly Recommends In Person Learning For The 2021 2022 School Year And Urges All Who Are Eligible To Be Vaccinated To Protect Against Covid 19 Read More Here

Covid 19 Information And Resources Tnaap

Covid 19 Vaccine Use In Children Conversation Aafp

Covid 19 Information And Resources Tnaap

American Academy Of Pediatrics On Twitter It S Finally Here Covid 19 Vaccines Are Now Available For Kids Ages 6 Months To 5 Years Vaccination Is The Best Way To Protect Your Child Callyourpediatrician
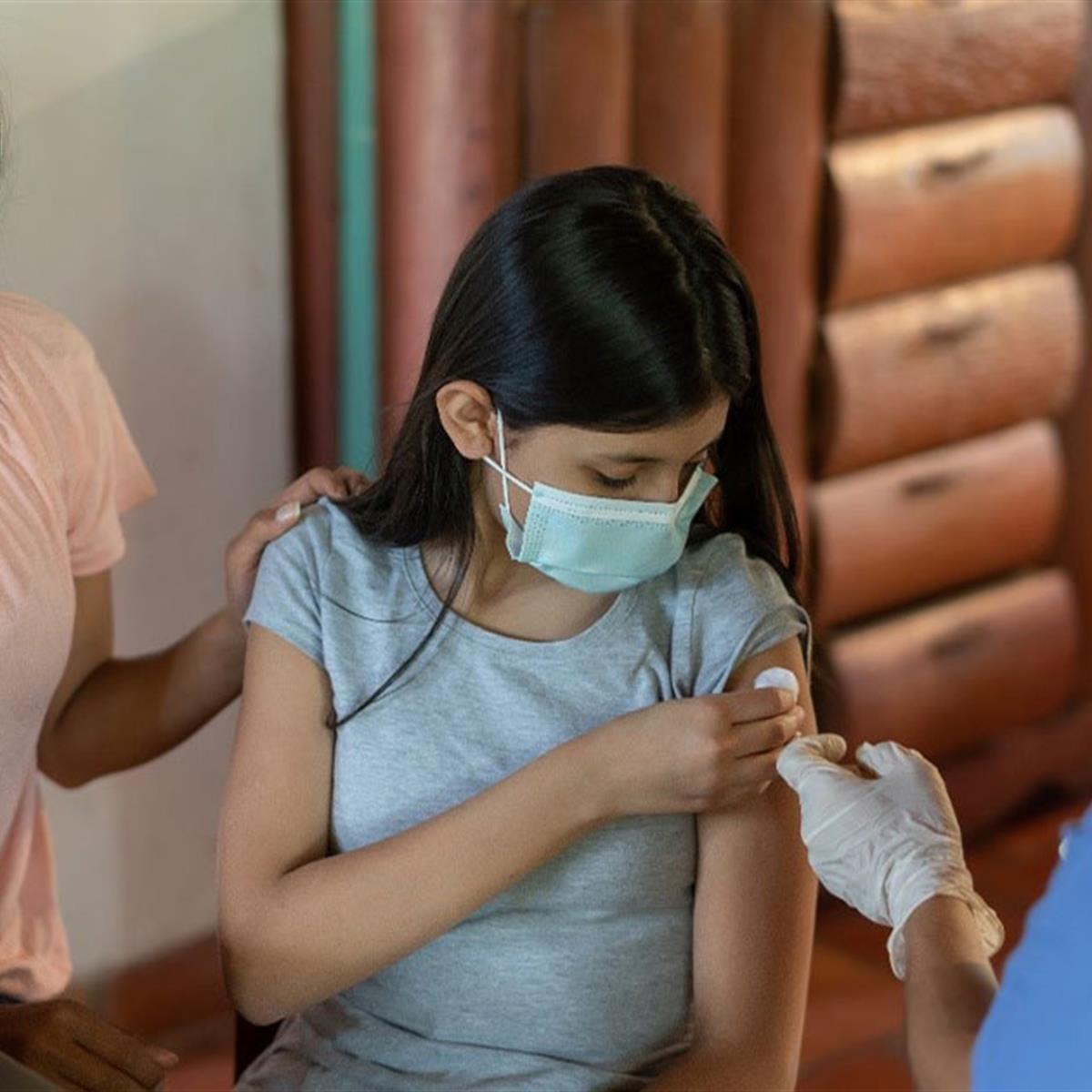
Aap Advises Children And Teens Age 12 And Up Get The Covid 19 Vaccine Healthychildren Org

Covid 19 Information And Resources Tnaap

Family Immunization Resources Illinois Chapter American Academy Of Pediatrics
